
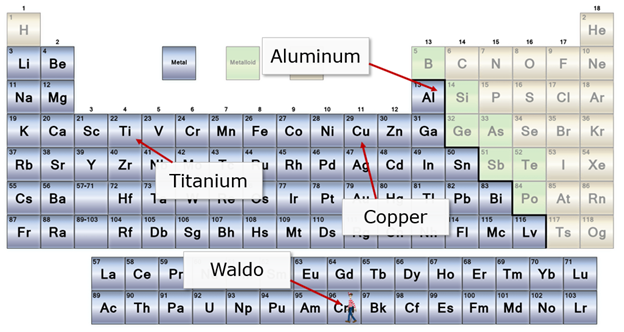
Last week (Steel: Why?), I wrote a lot about why steel was a good choice for swords. But what the heck is steel in the first place? I mean, you have a look at the periodic table and you see lots of friends like aluminum, copper, and titanium. But where is steel?
Enter the Alloy
Steel is something called an alloy. Basically, an alloy is a bunch of different metals melted together, and a convenient way to invent a science fiction super metal. Typically alloys will have a principal metal and a number of additives.
Iron + Carbon = Steel
Copper + Zinc = Brass
Copper + Tin = Bronze
Titanium + Aluminum + Vanadium = Titanium Alloy*
Steel + Vibranium = Adamantium**
*When people say titanium, they are actually talking about titanium alloy. Unlike Iron and Copper, which have uses of their own, we typically never actually see pure titanium in use.
**May not be real.
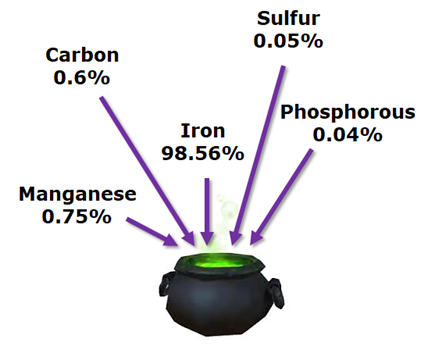
Now, this is just the main part of the alloy, typically you have a whole bunch of other stuff. For instance, ANSI 1060 Steel, — a typical sword steel — is specified to be:
Crystals!
Yes, the fantasy writers had it right all along. The quality of a steel all comes down to its crystals.
A metal is a collection of atoms all linked to each other in some sort of regular pattern. Depending on how they arrange themselves, the metal will have different properties. The whole structure is known as a crystal lattice, and it’s made up of a large number of repeating unit cells.
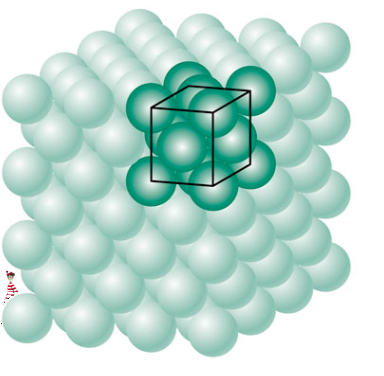
Iron is the primary metal in steel. If you have pure iron, all of the iron atoms are the same size and can form a nice organized crystal structure with good chakras. But what happens if we start throwing other junk like carbon and manganese in there? Bad feng shui!
The introduction of these impurities into the iron crystal lattice changes its physical properties. Exactly how it all works is a little technical for this article. But just take my word for it. (Or even better, start reading on your own.)
Grains is not as exciting a title as Crystals
A chunk of steel is not all one big solid crystal. As cool as this sounds, from a mechanical properties point of view, such a thing would not be an especially good idea. When chopping wood, you always set it up so that the axe is going along the grain. This is because a split can easily travel down the grain, requiring far less effort.

The same thing applies with metals. If you can hit it ‘along the grain’ you can fracture it much more easily.
Fortunately for us (well, those of use without dreams of karate chopping through steel plates), metals aren’t one solid crystal, but a collection of a whole bunch of grains stuck together. Less like a log, and more like that sawdust and glue stuff your kitchen cabinets are made of.
Grain Structure
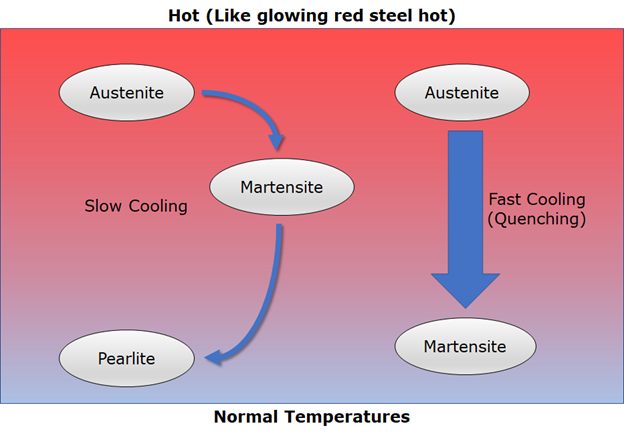
By controlling the alloy composition and the way the steel is heated and cooled, we can exercise a degree of control over the grains. Depending on temperature and alloy composition, you get different structures.
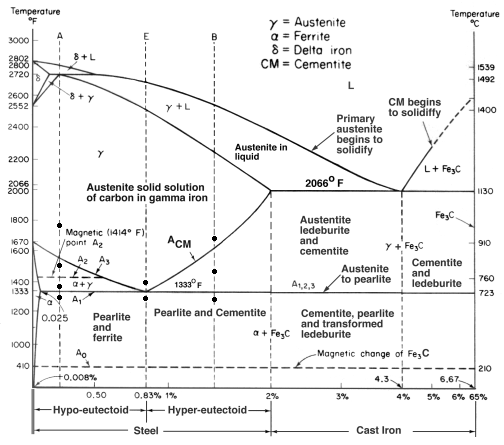
This is an oversimplification, but you can think of it this way:
1) Heated steel (glowing red hot) –> Austenite
2) As the steel cools it transforms from Austenite to Pearlite. The transition in the middle is a phase called Martensite.
3) If you cool the steel really quickly you effectively ‘flash freeze’ the Martensite in place, and it can’t transform into Pearlite.
What is the difference between Pearlite and Martensite? Naturally, being different crystal structures, they have different chakras. Just as important as chakra, the different crystal shapes result in different physical properties.
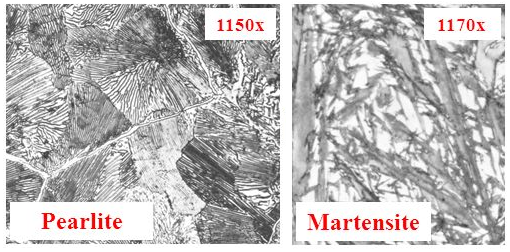
Notice that the pure Martensite is very ‘needley’. How well are needles known for flowing smoothly past one another? Not very. This makes Martensite very hard, which is why taking hot steel and quenching it is known as hardening.
On the other hand, because these grains can’t really move past each other, they tend to simply snap if too much force is put on them. The Pearlite, on the other hand, is softer because it has a little bit of play for grains to slide past each other without breaking apart. This means that while a sword with a Martensite structure would simply snap, a Pearlite structure would take a slight set but still be ok. This is toughness.
But what if we want a sword that’s both tough and hard? The key is to have both martensite and Pearlite in it! There are two ways of doing that: differential hardening, and tempering.
Differential Hardening
Differential hardening is when different parts of the sword are cooled at different rates, so that some parts of the sword are martensite and some parts are Pearlite. Most famous for this is the katana. The leading edge is kept the much harder martensite, forming a hard cutting edge. The larger part of the blade behind the lead edge is Pearlite, contributing toughness so that the brittle cutting edge is supported by something tough.

This is what forms the katana’s distinctive hamon, the boundary between the two grain structures. Conversely the two grain structures don’t exactly like to play nice with each other — that’s why if the sword ever does bend, it doesn’t like to go back to the way it was.

Tempering
Tempering is a much more common method of controlling steel properties. In fact, even deferentially hardened swords are tempered.
Recall that I said that the steel grains could be ‘flash frozen’ into having a Martensite structure. Martensite isn’t the normal structure steel wants to be in at room temperature, but it’s stuck there and doesn’t have enough energy to rearrange itself into a normal state.
Tempering is the practice of increasing the temperature just enough that the martensite slowly starts transforming into Pearlite, but controlling the whole process so that we end up with a blade that contains just the right proportions of Martensite and Pearlite. These phases are all mixed up together, indistinguishable without using a microscope.
Which is why a bad sword is often the result of heat treatment. Leave the sword too Martensitic and it is brittle and snaps easily. Convert too much martensite into Pearlite and the sword becomes soft and doesn’t stay sharp. Or, in the case of blunt swords, gets chewed up too easily.
This is also why advertising the type of steel shouldn’t be that much of a selling point. While there are certain steels that are completely unsuitable for swords (stainless steel, low carbon steels), you can take a fairly ‘ordinary’ steel like 1060 and make very good blades. And you can take more exotic spring or tool steels and produce far inferior blades due to a worse heat treatment process.
(I probably would have put a “Riddle of Steel” reference somewhere in this article, but I’ve never seen Conan and just don’t care. Bring the hate.)
Stuff For Nerds
Did any of these simplifications/creative licence descriptions irritate you? You sound like you have the knowledge to write a more detailed follow up piece. 😉
- Yes, I know that the ANSI steel specification is a range/maximum, not exact numbers like I threw into the cauldron.
- Yes, I know that fracture propagation in metals doesn’t look exactly like fracture propagation in wood.
- Yes, I know that comparing a steel microstructure to sawdust plus glue isn’t exactly correct, as it isn’t like the grains of steel are suspended in a matrix (well unless we are talking about a minor phase dissolved in a larger substrate phase…)
- Yes, I know that in equilibrium cooling the Austenite doesn’t transition through martensite. I feel it’s an acceptable compromise for the sake of clarity.
- Yes, I know that fractured steel can be examined for grain structure with a trained eye, and you can guess about the Martensite/Pearlite composition without a microscope.

